A Medical Milestone: The First Non-Opioid Drug for Severe Pain
How publicly-funded science, pharmaceutical companies, and the FDA brought us a new first-in-class non-opioid analgesic
Why was there an opioid addiction epidemic in America? One cause was Purdue Pharma’s aggressive efforts to convince physicians to freely prescribe a drug that the company knew was addictive. But another cause is that treating pain effectively is an unsolved problem. Millions of Americans suffer from not only acute pain, after surgery or an injury, but also debilitating chronic pain. For awhile, I was one of them. Terrible ergonomic habits at work caused me to develop severe neck and shoulder pain that lasted for years. If you had asked me, I would have rated my pain as at least a six out of ten on most days, and it was rarely better than that. Chronic pain is not just uncomfortable but distracting, making it difficult to focus on anything. I suffered, but my productivity also suffered, as it does for many people with chronic pain. Across the U.S., according to a 2012 study, the total annual cost of untreated pain, including both health care costs and lost productivity, is more than half a trillion dollars.
For a long time, the only drugs on the market to treat severe pain were opioids. Because they can be addictive, and because they lose their efficacy over time as people develop tolerance, opioids are not a solution for chronic pain, but people suffering from chronic pain take them anyway. I was once prescribed opioids after an outpatient procedure. I didn’t need them to recover from the procedure, but I took them anyway to get some relief from my incessant neck and shoulder pain. I stopped taking opioids when my prescription ran out, but others aren’t so lucky. In one study of Medicare patients, 10% continued to persistently use opioids after receiving a short-term prescription with a surgery. When you combine an unmet need for pain treatment with lax prescribing practices for an addictive drug, you get the origins of an opioid epidemic.
America’s opioid epidemic, now fueled by cartel-produced fentanyl, has developed beyond its origins in the abuse of prescription pain medicine. But the need for better drugs to treat severe pain is still unmet. That is why the FDA’s first approval of a non-opioid drug for severe pain is an impotant milestone. Approved at the end of January, suzetrigine (commercial name is Journavx, don’t ask me how to pronounce it) is a first-in-class, non-opioid analgesic from Vertex Pharmaceuticals, the company known for its breakthrough cystic fibrosis drugs. Suzetrigine was found to be effective for treating pain in two randomized, double-blind trials of patients who had undergone a surgical procedure. These trials looked at efficacy for acute, post-surgical pain, but ongoing trials will determine whether the drug is safe and effective for other indications, such as diabetic neuropathy and other forms of chronic pain. If it is, then this drug, and likely others in the pipeline, will transform how we treat pain.
Reaching this milestone is something that could only be accomplished on a deep foundation of neurobiology built with decades of publicly-funded research. The story behind Suzetrigine shows how enterprising and forward-looking industry research programs, working in tandem with academic scientists, can turn scientific knowledge into therapeutics that make people’s lives better.
Blocking pain before it reaches the brain
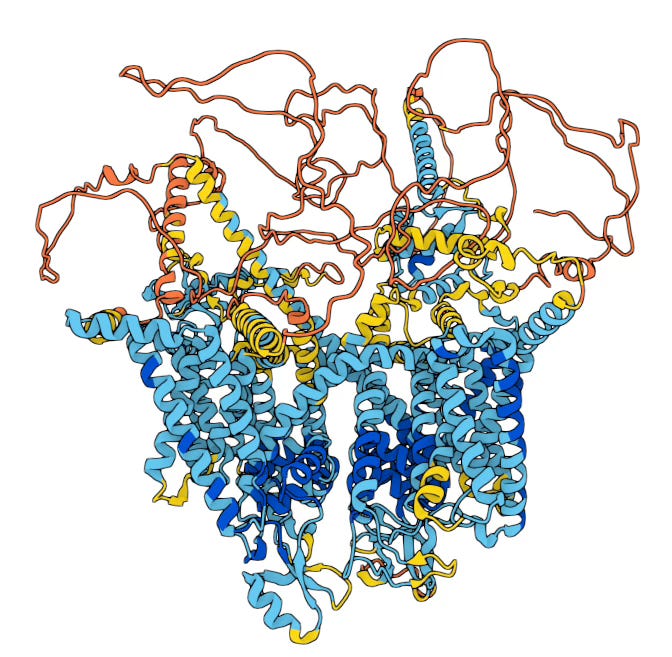
Suzetrigine is a small molecule that works by inhibiting a voltage-gated sodium channel called NaV1.8. Sodium channels mediate the electrical signaling of neurons by allowing sodium ions to pass through the cell membrane. (Wikipedia has a nice primer if you need it.) By blocking the action of NaV1.8 sodium channels, Suzetrigine prevents pain signals from being transmitted to the brain.
This sounds simple, but the contrast with opioids illustrates the challenge of inhibiting the neuronal signaling in very specific ways. Opioids act on µ-opioid receptors, many of which are located in pain-processing regions of the brain. Unfortunately, these receptors are also present in the reward systems of the brain, as well as in the brain stem, which controls our breathing, and in the small intestine. Hence, opioids not only affect our sensation of pain, but they cause euphoria, produce constipation, and depress breathing. In other words, opioids are dangerous because they aren’t selective enough for those parts of our nervous system responsible for signaling pain.
The history of the past few decades of pharmaceutical research for analgesics is basically a quest for drugs that are selective inhibitors of pain signaling. Suzetrigine is a medical milestone because it is highly selective for NaV1.8 sodium channels, which are almost exclusively present in the peripheral nervous system, and not the brain. By inhibiting sodium channels in the peripheral nervous system, primarily at the dorsal root ganglion where the peripheral nervous system connects with the spinal cord, Suzetrigine blocks pain signals before they reach the central nervous system. The drug thus leaves the pain and reward centers of the brain unaffected, and avoids the side effects that make opioids so dangerous.
Before we get into the scientific backstory, it is important to emphasize another important contrast between Suzetrigine and opioids. This is the difference between drugs that are developed to be specific using the molecular knowledge of modern science, and non-specific drugs based on a phenomenological understanding of medicines common in pre-scientific thought. 8000-year-old cuneiform tablets from Sumer describe the use of opium as medicine. For most of human existence, people discovered and used plant products as medical treatments without knowing anything at all about how they worked. While there are undoubtedly useful medicines that were discovered this way, all drug action happens at the molecular level. It is much more effective to develop drugs based on a molecular-level understanding of biology.
This may seem obvious, but we live in a society in which loud so-called health influencers with big platforms want to re-mystify biology and medicine by invoking pre-scientific theories like miasmas. Just like you can’t fix a car that won’t start unless you know the parts of the ignition system, we can’t develop medicines that address challenging medical conditions without detailed knowledge of the specific molecular interactions at the foundation of biology. The knowledge of those molecular interactions is the product of decades of publicly-funded research. In the rest of this post, I will walk through the important discoveries that made Vertex Pharmaceuticals’ success possible.
Discovery 1: Sodium channels
Alan Hodgkin and Andrew Huxley, at the University of Cambridge, discovered the existence of transmembrane sodium currents in neurons in 1952. They received the 1963 Nobel Prize for their fundamental work on the action potential of neurons. Bertile Hille (University of Washington) and Clay Armstrong (University of Rochester) began characterizing the properties of sodium channels in the 1960’s and 70’s, including how these channels were acted on by anesthetics. Daniel Beneski and William Catterall (University of Washington, supported by NIH grant HL-22234 and an NIH postdoctoral fellowship) characterized the protein components of sodium channels.
Over the subsequent decades, researchers discovered different subtypes of sodium channels, eventually identifying nine in humans. The gene for the subtype targeted by Suzetrigine, NaV1.8, was cloned from rat neural tissues in 1996 by two groups, one at University College London (funded by the Welcome Trust) and the other at Roche. Sodium channels are critical throughout the central and peripheral nervous systems, and different subtypes are expressed in different neural tissues. The discovery of NaV1.8, not expressed in the brain but rather in the peripheral nervous system, was an important advance.
Discovery 2: Link between NaV1.8 and pain
In 1999, the University College London group (led by John Wood) showed that mice missing the gene for NaV1.8 were less sensitive to pain. A University of Arizona group, led by John Hunter, showed a similar effect in rats. Three years later, this same group, in collaboration with Roche, showed that a suppressing the production of the NaV1.8 with an anti-sense oligonucleotide induced resistance to pain in rats. Further establishing the link between NaV1.8 and pain, an international group led by Stephen Waxman at Yale discovered that some people with painful neuropathies cary gain-of-function mutations in NaV1.8 (funded in part by Pfizer and by the U.S. Department of Veterans Affairs). Many other academic groups further characterized the role of NaV1.8 in pain signals in the peripheral nervous system, such as work led by Richard Carr and Matthias Ringkamp at Johns Hopkins University and the University of Heidelberg (supported by NIH grants R01NS09722 and F32DA036991.)
With the link between pain sensation and NaV1.8 established, Abbott laboratories, Pfizer, and others, developed molecules that specifically targeted NaV1.8, in the hope that these could lead to new analgesic drugs.
Discovery 3: NaV1.8 is expressed only in the peripheral nervous system
The critical piece of the success of Suzetrigine is that it selectively targets a sodium channel that is not expressed at significant levels in the brain or other non-targeted organs like the heart. It is this feature of NaV1.8 that made it the focus decades of research by both pharma and academic scientists, who knew years ago that a drug that could successfully inhibit only NaV1.8 would almost certainly avoid the dangerous side effects of opioids.
The specific expression of NaV1.8 in the peripheral nervous system was established early on. But two important publicly funded human expression atlas projects were cited by Vertex in their clinical trial report as evidence of the expression pattern of NaV1.8: the Human Protein Atlas and GTEx, two publicly funded projects that set out to comprehensively catalog the distribution of every gene and protein among human tissues. The knowledge of where in the body each gene and protein is expressed is crucial for developing selective drugs that act only where you want them to act.
Converting knowledge into therapy
Fundamental knowledge of sodium channels, and NaV1.8 specifically, is what enabled pharma scientists to choose NaV1.8 as an attractive target for drug development. Vertex Pharmaceuticals tested several compounds and discovered one that was much more selective than the rest. Other companies also have NaV1.8 inhibitors in development. Getting a working drug across the finish line is difficult, even with a deep foundation of molecular knowledge, in part because our knowledge is always imperfect. One reason, though not the only one, that drug development is so expensive is that the failure rate is high. An improvement in success rates for taking a drug from pre-clinical work to FDA approval will help bring down the cost producing new drugs. One of the most effective ways to improve success rates is to base drug discovery on genetic knowledge, most of which is produced by publicly funded academic scientists. A 2019 analysis found that using human genetic evidence to target drug development increased approval rates by more than two-fold. The story of Suzetrigine illustrates why. Extensive evidence in model organisms and in human patients with NaV1.8 mutations established a strong scientific foundation for a drug development program.
The final important piece of drug development is the regulatory agency. The FDA has an obligation to keep unsafe and ineffective drugs off the market, but equally important is its mission to facilitate the release of drugs that are safe and effective. The agency established important guidelines and processes for drugs that could treat urgent, unmet medical needs, and suzetrigine was approved under the FDA’s Breakthrough Therapy, Fast Track, and Priority review pathways.
The opioid epidemic was driven in part by the criminal activity of one pharmaceutical company, but a major, unmet medical need was another important driver. There are still many unmet medical needs that could be solved with better drugs. Those future drugs will, more often than not, emerge from the fertile soil of our rapidly growing, publicly funded stock of genetic and genomic knowledge.
If you want to learn more about the development of NaV1.8 sodium channel inhibitors, The New England Journal of Medicine’s podcast Intent to Treat put out an informative episode with Yale neurobiologist Stephen Waxman back in 2023, when the suzetrigine study was published. Waxman has long been one of the leaders of the NaV1.8 field.

