Measuring long distance communication in the genome
When two methods give you different answers don't give up because that's when things get interesting
One of the keys to success in life with large genomes is long distance communication. Regulatory information is widely distributed in the genome, and thus to express the right genes in the right cell types at the right times involves bringing linearly distant parts of a chromosome close together in 3D space. Regulatory DNA elements like enhancers may be thousands or millions of base pairs away from the genes they act on, but they nonetheless end up physically close to their targets when the action happens.
What’s not clear however is just how close enhancers need to be to their targets. The mechanistic details of gene regulation by enhancers are still not well understood, and there is still an active debate in the field over whether regulatory DNA elements need to physically contact their targets in order to function. Resolving this debate isn’t merely a matter of scientific curiosity — genetic variants in regulatory DNA appear to have pervasive effects on human phenotypes. Understanding how regulatory DNA functions, and how that function is altered by genetic variation is therefore a major research priority.
One common approach to determine whether two segments of DNA are in contact is to use chemical crosslinkers to glue physically close segments together. The crosslinked pieces are then identified by sequencing, and the data is used to create large-scale maps of 3D genomic proximity. An alternative way to determine whether two DNA segments are touching is to use imaging. Two DNA segments can be labeled with fluorescent probes, and the distance between the probes measured under the microscope. Unlike crosslink-and-sequence techniques, fluorescence microscopy is low throughput because generally only measure one pair of DNA locations at a time.
In science, it’s good to have multiple ways of measuring something you care about, because when the methods agree it gives you confidence in the result. But it’s also good when methods disagree, because then you’ve uncovered an interesting problem that could lead to a better understanding. As it turns out, measurements of enhancer-target proximity often disagree, and so we don’t always know whether an enhancer is physically contacting its target during the process of regulation. A new paper in PLOS Genetics presents an elegant set of experiments that helps resolve the sometimes contradictory measurements of crosslink-based and microscopy-based methods.
Wendy Bickmore’s lab, at the MRC Human Genetics Unit at the University of Edinburgh, examined several inducible enhancers to tackle the question of how close enhancers need to be their target. The Bickmore lab used inducible enhancers because that allowed them to see how the enhancer-target distance changes between the uninduced, off state and the induced, on state. The idea behind the experiments is simple: Use both microscopy and cross-linking/sequencing to measure the change between the off and on states. Then make some careful perturbations to the enhancer to tease out key differences between the measurements.
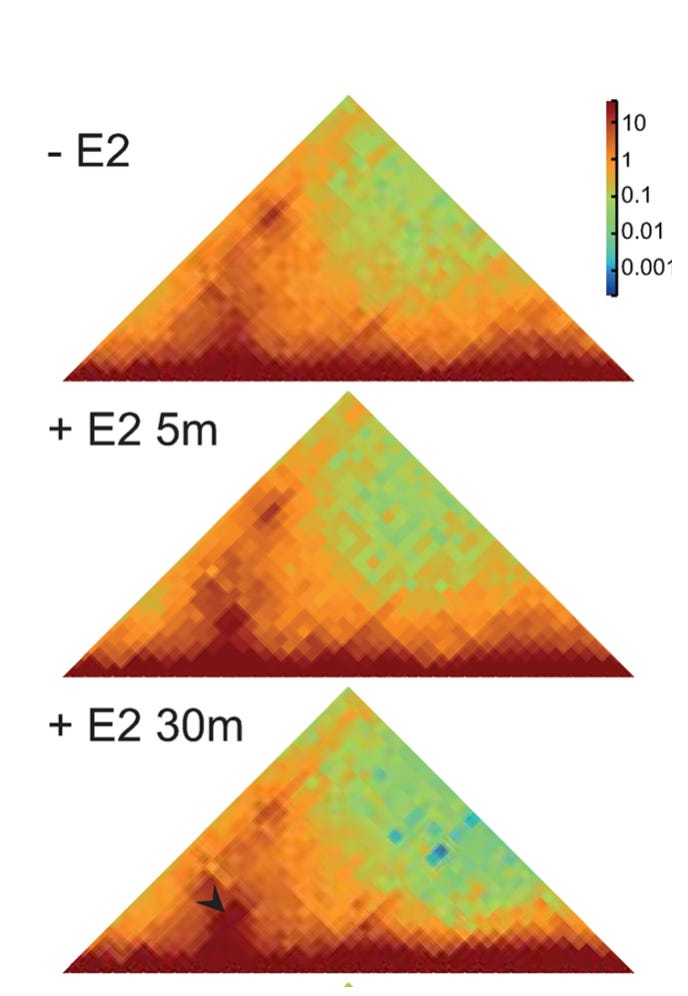
Crosslink-based measurement of induced contact. Figure 2A from Gómez Acuña, et al., PLoS Genet 20(5): e1011277.
Their first experiment demonstrates the paradox: Crosslinking shows that, upon induction, the enhancer increases its contact frequency with its target. Microscopy however shows that induction causes the enhancer to move away from its target. More specifically, they found that after induction, the enhancer was more likely to be > 200 nm away from its target than it was before induction. This is the basic discrepancy that needs to be resolved.
Microscopy shows increased distance between enhancer and promoter after induction. Figure 2C from Gómez Acuña, et al., PLoS Genet 20(5): e1011277.
The key experiment of the paper manages to resolve it by identifying conditions under which contact frequency increases (measured by crosslinking) while nothing changes as measured by microscopy. The Bickmore lab did this by blocking the regulatory function of the enhancer with a drug that inhibits active transcription. When they induced the enhancer in the presence of the drug, the enhancer still increased its contact frequency with its target. But under the microscope, the researchers no longer observed the enhancer moving away from its target.
So what’s going on? The Bickmore lab interprets their results as showing a two-state mechanism of enhancer action that works as follows:
Before induction, the enhancer and its target are already somewhat close in 3D space (as seen in the images of the uninduced cells), but not close enough to be detected by cross-linking.
Induced state 1: Upon induction, proteins bind the enhancer and its target and bring them close together (as detected by crosslinking).
Induced state 2: As transcription gets underway, the enhancer moves away from its target as a larger collection of regulatory proteins arrive and perhaps create a large biomolecular condensate around the active site of transcription.
In other words, bringing the enhancer close to its target is a separable event from active transcription of the gene - you can have one without the other. During active transcription, enhancer and target don’t necessarily need to be particularly close, perhaps because there is a large molecular apparatus bridging the gap between them.
While this is an interesting result for those of us interested in gene transcription, the broader lesson is the scientific version of Yogi Berra’s famous piece of advice, “When you come to a fork in the road, take it.” When you find different experimental techniques giving you different answers, embrace them both because they’ll lead you to new insights.